Solid State Ion Conductors
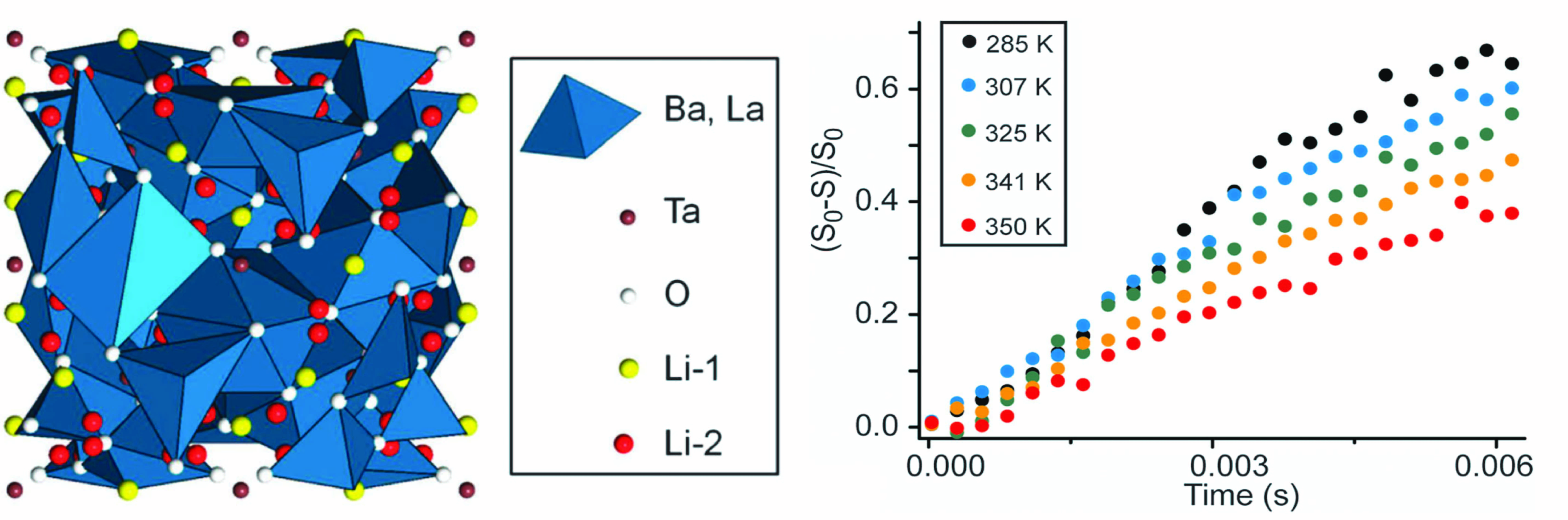
In lithium ion batteries, the implementation of a solid electrolyte would allow for the use of high energy density Li metal anodes, simultaneously decreasing the flammability caused by the use of volatile organic electrolyte solvents in commercial cells. In the Goward group we use solid-state NMR to analyze the lithium ion conductivity in a number of solid-state Li ion conductors, such as Li6BaLa2M2O12(M = Ta and Nb), where we are able to observe lithium motion both form the point of view of the mobile ion as well as the framework atoms. Inparticular, we have used 6Li{7Li}-REDOR to study the effective dipolar coupling in garnet structures in order to directly probe the mobility of the mobile Li ion. Changes in the slope of the resulting REDOR curve are correlated to ionic hopping rates of lithium ions, and variable temperature studies can be used to determine the affect of temperature on ion mobility. We have also been able to observe the stationary framework elements, such as 139La, to observe ionic motion from a second point-of-view. Here, changes in the quadrupolar lineshape are correlated to lithium ion hopping, and spectral simulations are used to extract these rates. Together, these methods provide multiple perspectives from which to measure inherent lithium ion motion in solid-state electrolytes.
The use of a solid-state proton conductor in intermediate temperature fuel cells (100-400 °C) has the potential to improve the overall efficiency and therefore lower the cost, of traditional proton exchange membrane fuel cells. Solid-state proton conductors tend to be complex, often containing many proton environments that can participate in multiple proton-proton interactions and form complex transport pathways. Our work in this area focuses on the use of site-selective solid-state NMR techniques that allow us to probe these proton environments individually by measuring dipolar coupling and ion hopping rates that allow for the elucidation of the proton transport mechanism in these materials. The NMR data can then be compared to bulk proton conductivity, measured via electrical impedance spectroscopy, to demonstrate the contributions of individual proton transport pathways to this macroscopic property.